Beneficence Belmont Report
A report issued by the US. The ethical obligation to.
Chapter 3 was expanded by adding a definition of anonymity elaborating on the Belmont Report the principles of respect for persons and beneficence were added and adding a link to a clip dispelling the myth that vaccines cause autism.

. An Ethics Advisory Board was formed in the late-1970s to review ethical issues of biomedical research. Right to Treatment Informed Consent Confidentiality. What is the Belmont Report.
The Belmont Report beneficence principle is the obligation of a researcher to do no harm to a participant. Its primary purpose is to protect subjects and participants in clinical trials or research studies. Similarly the three ethical principles laid forth in the Belmont Report ask providers to prioritize respect for persons beneficence and justice in their daily practice.
The term beneficence is often understood to cover acts of kindness or charity that go beyond strict. The Hippocratic Oath instructs physicians and other medical providers to first do no harm. Many of these guidelines are based on the Belmont Report pdf.
It is the outgrowth of an intensivefour-day period of discussions that were held in February 1976 at. National Center for Biotechnology Information. Ethical Considerations and Advances in the Understanding of Animal Cognition.
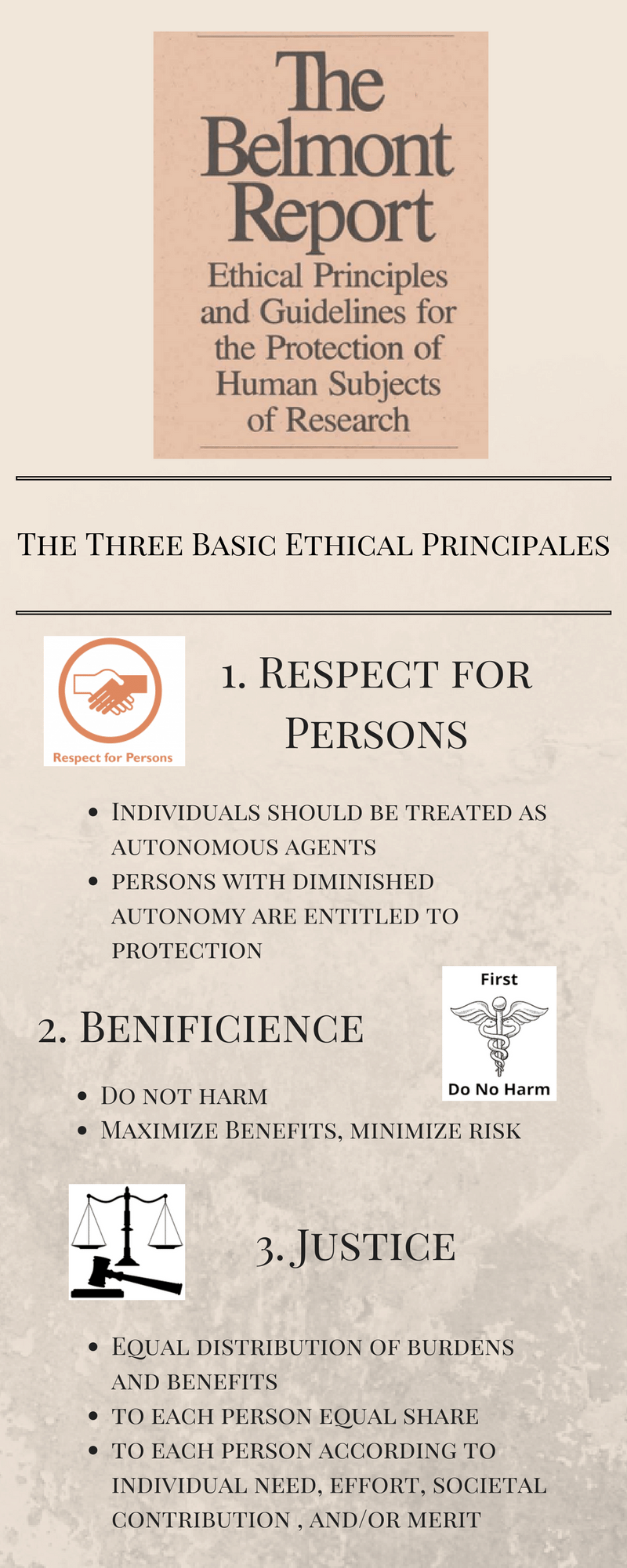
Belmont Report National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research 1979 which identified the principles of respect for persons beneficence and justice in human subjects research. This commission was established in 1974 by the US. The basic ethical principles.
In 1981 Beauchamp and Childress built on this work and applied it to health care in the first edition of their book. Sections from Chapter 4 on defining theories and hypotheses were moved to Chapter 2 and the remainder of. Researchers must minimize harm to research participants and maximize benefits of research.
While is it critical for providers of medical care to uphold these tenets frequently situations arise where it is. National Commission for the Protection of Human Subjects in Biomedical and Behavioral Research in 1979 which has had a significant influence over human subjects research ethics. This report consists of 3 principles.
In its 1978 Belmont Report the Commission stipulated that in reviewing research proposals IRBs should be guided by three basic ethical principles. The guiding ethical principles of the IRB - respect for persons beneficence and justiceare embodied in the Belmont Report. The Belmont Report attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations.
Ethical Principles and Guidelines for the Protection of Human Subjects of Research The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research April 18 1979. The Belmont Report attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations. Whereas beneficence refers to actions or rules aimed at benefiting others benevolence refers to the morally valuable character traitor virtueof being disposed to act to benefit others.
Apprehension around burgeoning medical research in the late 1800s and the first half of the 20 th century sparked concerns over the use of humans and animals in research Suspicions around the use of humans were deepened with the revelation of several exploitive research projects. Justice is the ideal distribution of risks and benefits when scientists conducting clinical research are recruiting volunteer research participants to participate in clinical trialsThe concept gives guidelines on how scientific objectives and not membership in either a privileged or vulnerable population should. Following a series of highly-publicized medical and behavioral research scandals the 1974 National Research Act and 1979 Belmont Report set a new agenda for research ethics in the US that rested on principles of beneficence respect for persons and justice.
Beneficence -- requires that experiments do not harm research subjects and that researchers minimize the risks for subjects while maximizing the benefits for them. Study with Quizlet and memorize flashcards containing terms like Which of the following are the three principles discussed in the Belmont Report Which of the following is an example of how the Principle of Beneficence can be applied to a study employing human subjects All of the following are true regarding the Belmont Report EXCEPT. Patients should always benefit from medical interventions.
Ethical Issues Mental Health. These include guidance documents and frequently asked questions FAQs addressing various topics findings in the form of OHRP letters addressing regulatory issues and other media including. OHRP has published a variety of policy and regulatory guidance materials to assist the research community in conducting ethical research that is in compliance with the HHS regulations.
In research ethics justice is the fair selection of research participants. Respect for persons beneficence and justice. First that individuals should be treated as autonomous agents and second.
To interpret these ethical principles IRBs would ultimately look to an unlikely amalgam of concerned healthcare professionals scientists. Subsequent regulations through the Department Health and Human Services require all. Study with Quizlet and memorize flashcards containing terms like Which of the following are the three principles discussed in the Belmont Report an example of how the Principle of Beneficence can be applied to a study employing human subjects incorporates at least two ethical convictions.
As a result of their work the 1979 publication commonly known as The Belmont Report external icon summarized the three ethical principles that should guide human research. Human subject research とはヒトを対象とする研究体系的な科学的調査でありヒトを研究主題として含んだものを指す. The Belmont Report is one of the leading works concerning ethics and health care research.
It is the outgrowth of an intensive four-day period of discussions that were held in February 1976 at the Smithsonian Institutions Belmont Conference Center supplemented by the monthly deliberations of the. The Belmont Report has provided the basic moral framework for research ethics in the United States.
Historical Context And Economic Issues Ethical Legal And Societal Implications Of Biotechnology

From The Belmont Report To The Code Of Federal Regulations

Comparison Of The Application Of The Respect For Persons Principle As A Download Scientific Diagram

Extensions Of The Belmont Report S Principles Based On The Guidelines Download Scientific Diagram

0 Response to "Beneficence Belmont Report"
Post a Comment